Possible Reduction of Cerebral Palsy with Novel Strategies
Kazuo Maeda
DOI10.21767/2471-9749.100042
Kazuo Maeda*
Department of Obstetrics and Gynecology, Tottori University, Yonago, Japan
- *Corresponding Author:
- Kazuo Maeda
Department of Obstetrics and Gynecology
Tottori University, Yonago, Japan
Tel: 81859226856
E-mail: maedak@mocha.ocn.ne.jp
Received Date: February 05, 2018; Accepted Date: February 13, 2018; Published Date: February 20, 2018
Citation: Maeda K (2018) Possible Reduction of Cerebral Palsy with Novel Strategies. J Contracept Stud Vol.3 No.1:9
Abstract
Intrapartum brain damage followed by cerebral palsy developed after the loss of fetal heart rate variability and new hypoxia index was 25 or more, thus, early delivery will be done before these CP predictive borders in fetal monitoring. Very early sign of uterine contraction should be detected to cease preterm labor reducing neurological sequels. Neonatal brain PVE should be rejected after preterm birth reducing PVL and CP. The respiratory distress syndrome is predicted for 96% by ultrasonic GLHW fetal lung tissue characterization, reducing hypoxic neonatal disorder of central nervous system.
Keywords
Fetus; Cerebral palsy; Fetal heart rate variability; Fetal movement; Periventricular echodensity; Leucomalasia
Introduction
Infantile cerebral palsy (CP) was constantly 0.2 to 0.25% and did not reduce for 30 years in a 2003 report [1], particularly the CP incidence was high in low birth weight preterm infants, while the CP reduced after full external fetal heart rate (FHR) monitoring in a general hospital [2], and pediatric research of whole deliveries in Tottori area [3] revealed 0.25% of CP incidence (98/40,532 births) in 1960 before introduction of fetal monitoring and significant reduction to 0.06% (29/50,814) in 1980 after wide spread of fetal monitoring.
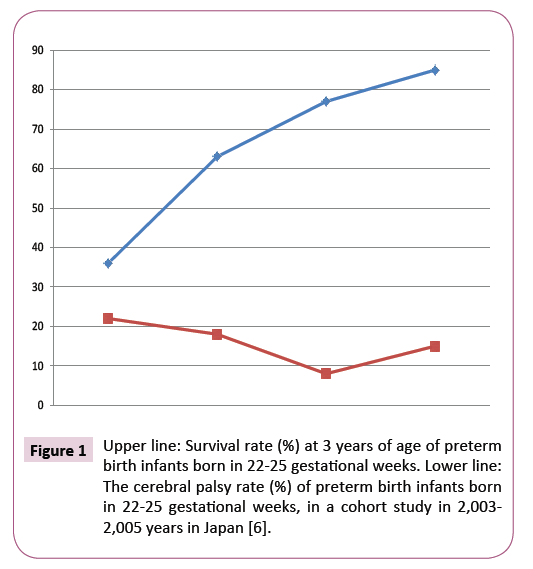
Recently, there are trends of CP decrease in very low birth weight and preterm birth infants in the reports in 2007 [4,5]. The survival rate was high in the babies born in 22 to 25 weeks, while there were considerable number of CP cases (Figure 1) [6].
Since fetal brain damage was studied in the FHR response to fetal movement using actocardiogram [7,8], relations of fetal periventricular echo density (PVE) and neonatal periventricular leucomalacia (PVL) [9], developmental mechanism of labor contraction [10], and the gray-level histogram width (GLHW) ultrasonic tissue characterization have been progressed [11-15], some new strategies probably to decrease the CP are proposed in the present report as follows.
Materials and Methods
The loss of FHR variability will be the sign of general fetal brain damages
The loss of FHR acceleration in the non-reactive FHR preserving FHR variability is early sign of fetal hypoxia, then heavy hypoxia characterized by the loss of variability resembles fetal anencephaly, brady cardia and severe late deceleration appear (Figure 2) some days later than the loss of acceleration (nonreactive state) in fetal hypoxia, which will promote early caesarean (C-) delivery preventing fetal brain damage, while the C-delivery after the loss of FHR variability will prevent fetal demise but will not prevent fetal brain damage followed by CP. The C-delivery is recommended prior to the loss of variability, which is the sign of fetal brain damage by severe hypoxia, because the variability is the reaction of fetal brain to minor fetal movements [7,8].
The author recommends to receive rapid and urgent C-delivery before the loss of variability, which will be predicted by the loss of FHR acceleration (non-reactive state), and by the other hypoxic signs, e.g., bradycardia, repeated severe FHR decelerations, high FHR score of 15 or more points, the reduced variability less than 5 bpm, or high hypoxic index (20-24 points) calculated by the sum of bradycardia durations (min) divided by the lowest FHR, and multiplied by 100 [16,17], while the loss of variability followed by fetal brain damage and cerebral palsy will appear, if hypoxia index is 25 or more.
The periventricular leucomalasia (PVL) followed by CP, which were preceded by fetal periventricular echodensity (PVE)
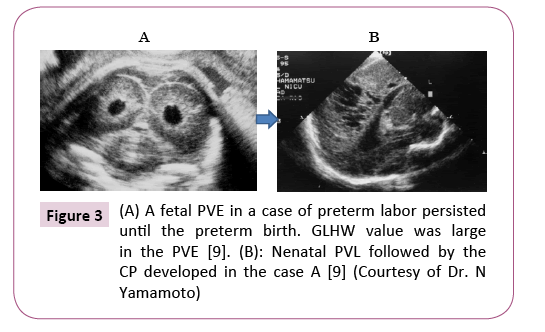
The PVL did not appear in full term birth infants and in cases of disappeared PVE before preterm births, while 18% of cases, who preserved PVE before the preterm births, developed neonatal PVE followed by PVL and CP, which will be 4 cases in approx. 2,000 births (0.2%) (Figure 3) [9].
Treatment of preterm labor
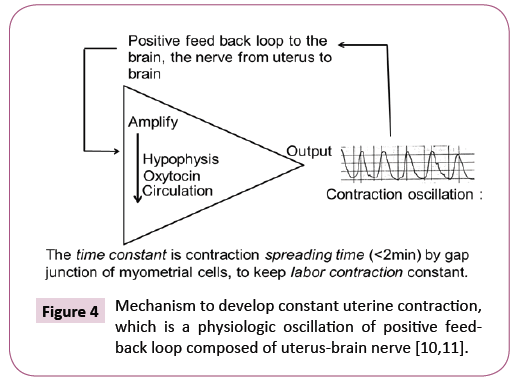
A strategy will be the tocolysis to delay the births until full term birth in the management of fetal PVE, namely, the tocolysis with ritodrine is common, while a novel tocolys mayl be the suppression of maternal uterus-brain nerve [10,11] in the positive feed-back loop to develop regular labor contraction [12], where the sedative methods, e.g., analgesia or anesthesia applied to sedate preterm labor, where the developing mechanism of uterine contraction in the preterm labor will be confirmed using uterine contraction records before treatment, as it is explained in Figure 4.
As the regular uterine contraction is a perfect oscillation of uterine contraction with the intensity saturation of feed-back loop, achieved by repeated excitations of positive feed-back loop, thus contraction intensity will be strong enough, and sufficient to resist usual tocolysis, then usual doses of terbutaline will be failed to stop preterm labor. Thus, terbutaline will be effective in very early stage of weak preterm labor, possibly the first or second excitation of feed-back loop, where withhold be treated by tocolysis. The timing will be found in careful monitoring of pregnant uterus in very early stage. The analysis of Braxton-Hicks contraction, which starts around 30 weeks of pregnancy, but does not achieve fetal delivery. Thus, it will be very useful to study Braxton-Hicks contraction for the treatment of preterm labor.
Treatment of neonatal brain PVE
Preserved PVE in the preterm fetus until the preterm birth will be detected in the day of preterm birth by detailed B-mode study of preterm neonatal head, and it has to be treated to erase before the change to PVL followed by CP, by un-established treatments possibly by hydrocortisone, erythropoietin or growth factor. The last one was rich in fetal blood in late stage of pregnancy and disappeared in the neonatal blood in 3-4 days after birth [13].
Prediction of neonatal respiratory distress syndrome (RDS)
The immature fetal lung is detected with GLHW, a clinical ultrasound tissue characterization, by using common real-time B-mode machine [14].
GLHW detected grade 3 placenta, fibrin deposit in placental intervillous space, malignancy of endometrium and ovary, turbid amniotic fluid, fetal brain PVE, and immature fetal lung which was the prediction of neonatal respiratory distress syndrome (RDS). The ratio of fetal lung GLHW and that of fetal liver was multiplied by gestational weeks, and fetal lung immaturity was detected when the calculated value was less than 29, and 96% of neonatal RDS was predicted in intrauterine fetus with 96% of sensitivity and 72% of specificity, respectively (Figure 5) [15].
The steroid therapy are repeatedly monitored by GLHW, if fetal lung immaturity was detected and received steroid to the mother, to achieve fetal lung maturity preventing neonatal RDS and hypoxia, because the GLHW is non-invasive and safer than the fetal lung maturity diagnosis with amniocentesis.
The tissue characterization with GLHW will be applied in general medicine, e.g. hepatic diseases, breast and the other malignant neoplasia or the other important tissue changes with common B-mode machine without particular computer or program. Aloka B-mode device prepares automatic calculation of GLHW value by %w in its histogram analysis [14].
CP rate in fetal disorders
It is told that there are congenital CP developed in fetal stage and partly in preterm labor, namely preterm labor and fetal disorders would be overlapped in the statistics of CP rate. How many fetal disorders will cause CP? It will be estimated by a study on the relation of short and long term outcomes and actocardiographic acceleration duration ratio to fetal movement burst duration (A/B) ratio. The outcome was unfavorable when the A/B ratio was <1.0 [16]. The long term outcome would be related with the CP, because a case of spastic quadriplegia was found in bad long-term outcome infants, where bad long-term outcome rate, which would be the same as CP rate was 0.19% (7/3600 births in 6 hospitals), and fetal disorder rate was 0.5% (20/3600) [16].
Results
The CP developed after intrapartum brain damage will be the incidence of fetal brain damage followed by cerebral palsy is around 1 in 5,000 births, thus, the number of CP will be 200 in 1,000,000 births in a year in Japan. The CP rate caused by preterm births are 0.2%, which is close to CP rate due to intrapartum damage in a year in Japan, thus, preterm delivery should be treated with novel techniques in the future, also.
Discussion
How will be the role of fetal disorders, which were reported to be main cause of CP? The number of fetal disorders were 20 in the study of fetal outcome in 6 hospitals [16]. Total number of births was 3600 in a year in the report [16], where the outcome was studied with the actocardiographic A/B ratio. The long-term fetal outcome was unfavorable when the A/B ratio was <1.0 in 0.19% (7/3600), i.e., 35% (7/20) would be CP in long-term outcome, and 1000,000 x 0.19 /100=1.900 had probably CP due to fetal disorders in a year in Japan, of which most part may overlap to preterm birth. The results will mean that preterm birth and common fetal disorders were main cause of 2,000 CP cases. National CP development will be remarkably decreased, if the CP developing causes including the loss of FHR variability and preterm births are reduced to half by the new strategies.
Thus, CP, 0.1 or more % of total births, would be caused by prenatal fetal disorders, of which reduction will be greatly improved in the future by the progress of fetal therapy [17], stem cell therapy in developed CP [18], chromosomal technology [19], general advances of obstetrics, pediatrics and general medical sciences focused on the reduction of CP.
Conclusion
The cerebral palsy caused by intrapartum fetal brain damage will be cured by timely caesarean delivery decided with novel fetal heart rate monitoring, as well as fetal lung immaturity is detected by noninvasive tissue characterization to contribute neonatal health and disorder treatments.
References
- Clark SL, Hankins GDV (2003) Temporal and demographic trends in cerebralpalsy-fact and fiction. American Journal of Obstetrics and Gynecology 188: 628-633.
- Tsuzaki T, Sekijima A, Maeda K(1990) The survey on the perinatal variables and the incidence of cerebral palsy for 12 years before and after the application of the fetal monitoring systems. Acta Obstetrics and Gynaecologica Japonica 42: 99-105.
- Takeshita K, Ando Y, Takashima S(1989) Cerebral palsy in Tottori, Japan. Benefits and risks of progress in perinatal medicine. Neuroepidemiology 8: 184-192.
- Platt MJ,Cans C, Johnson A, Surman G, Topp M, et al. (2007)Trends in cerebral palsy among infants of very low birth weight (more than 3.3 pounds) or born prematurely (less than 32 weeks) in 16 European centers: a database study. The Lancet 369: 43-50.
- Hack M, Costello DW (2007) Decrease in frequency of cerebralpalsy in preterm infants. The Lancet 369: 7-8.
- Ishii N, Kono Y, Yonemoto N (2013) Outcomes of infants born at 22 and 23 weeks’ gestation. Pediatrics 132:62-71.
- Maeda K (2012) Actocardiographic analysis of fetal hypoxia detected by the bradycardia, loss of fetal heart rate acceleration and long term variability. Journal of Health Medical Informatics 4: 1-5.
- Maeda K (2014) Origin of the long-term variability and acceleration of FHR studied for the prevention of cerebral palsy in fetal hypoxia and general insults. Journal of Perinatal Medicine 42:401-403.
- Yamamoto N, Utsu M, Serizawa M, Ohki S, Murakoshi T, et al. (2000) Neonatal periventricular leukomalacia preceded by fetal periventricular echodensity. Fetal Diagnosis and Therapy 15: 198-208.
- Gnanamanickam GJ, Liewellyn-Smith IJ (2011) Innervation of the rat uterus at esterus: a study in full-thickness, immunoperoxidase-stained whole-mount preparations. Journal of Comparative Neurology 519:621-643.
- Maristela OP, De’Nise TM, Raphael ES, Richard B, Cleyde VV, et al.(2012) Cervical stimulation activates A1 and locus coeruleus neurons that project to the paraventricular nucleus of hypothalamus. Brain Research Bulletin 88: 566-573.
- MaedaK (2013) Uterine contractions in normal labor developed by a positive feed-back and oscillation. Journal of Health and Medical Informatics 4:1-3.
- Tamaya T. Personal communication, 2000.
- Maeda K, Utsu M, Kihaile PE (1998)Quantification of sonographic echogenicity with gray level histogram width: a clinical tissue characterization.Ultrasound in Medicine and Biology 24: 225-234.
- Serizawa M, Maeda K (2010) Noninvasive fetal lung maturity prediction based on ultrasonic gray level histogram width. Ultrasound in Medicine and Biology 36: 1998-2003.
- Maeda K, Iwabe T, Yoshida S, Ito T, Minagawa Y,et al.(2009) Detailed multigrade evaluation of fetal disorders with the quantified actocardiogram. Journal of Perinatal Medicine 37: 392-396.
- Maeda K (2014) Modalities of fetal evaluation to detect fetal compromise prior to the development of significant neurological damage.Journal of Obstetrics and Gynecology Res 40: 2089-2094.
- Wang S, Cheng H, Dai G, Wang X, Hua R, et al.(2013) Umbilical cord mesenchymal stem cell transplantation significantly improves neurological function in patients with sequelae of traumatic brain injury.. Brain Research 1532:76-84.
- Inoue T, Shinohara T, Takehara S, Inoue J, Kamino H,et al.(2000)Specific impairment of cardiogenesis in mouse ES cells containing a human chromosome 21. Biochemical andBiophysical Research Communications 273: 219-224.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences